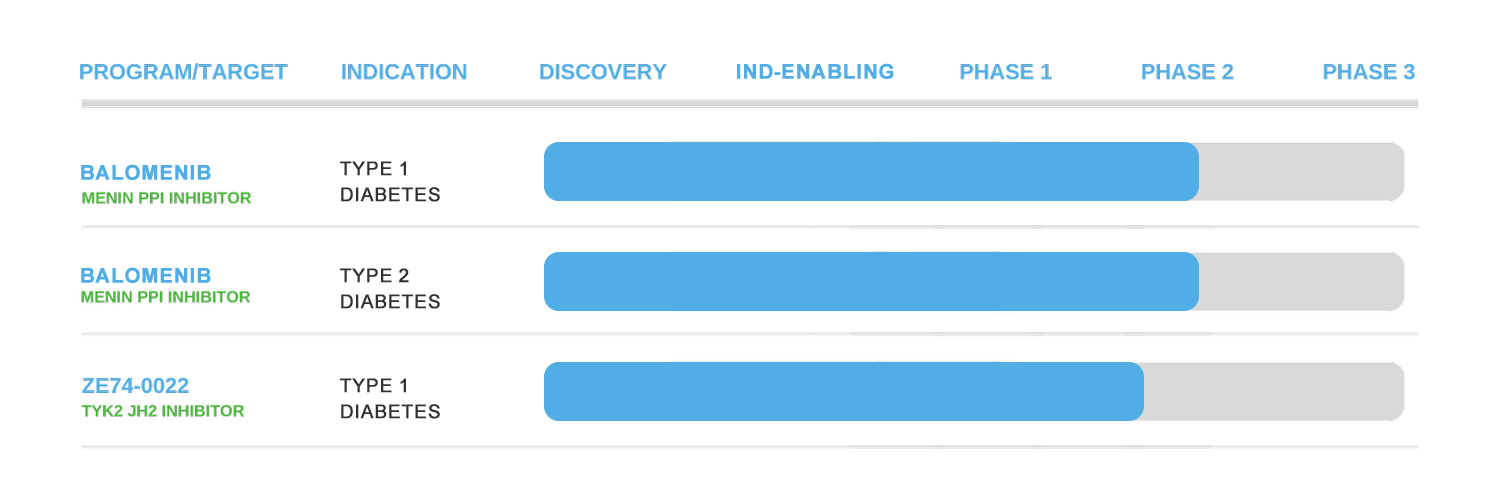
Pipeline
Clywedog Therapeutics Inc is a clinical-stage biopharmaceutical company developing first and best in class medicines to treat diabetes.
Co-founded and seed-funded by Orbimed Advisors and Torrey Pines Investment, with additional support from the Expert Systems hybrid AI/ML drug discovery and development accelerator.
Balomenib — First-in-class Menin PPI Inhibitor for Diabetes
Clywedog is developing Balomenib, a best-in-class, oral, reversible menin protein–protein interaction (PPI) inhibitor for the treatment of type 2 diabetes (T2D) and type 1 diabetes (T1D).
Mechanism in T2D
Menin normally binds transcriptional partners (e.g., JunD, MLL) to repress β-cell proliferation and function.
By blocking these interactions, menin inhibitors lift transcriptional repression, leading to:
β-cell regeneration
Enhanced insulin secretion
Improved glucose control
Unlike standard glucose-lowering agents, menin inhibitors represent a potential disease-modifying therapy by directly restoring β-cell capacity.
Synergy with GLP-1 Agonists
GLP-1 signaling phosphorylates menin at Ser487, sequestering it away from chromatin and transiently boosting insulin gene expression.
Menin inhibitors act pharmacologically and durably by preventing menin from recruiting epigenetic repressors.
Together, these mechanisms may be:
Additive — stronger or longer-lasting insulin expression
Complementary — GLP-1 agonists drive acute secretion, while menin inhibitors rebuild β-cell mass and function
Therapeutic implication: Menin inhibitors may prime β-cells for GLP-1 responsiveness, while GLP-1 agonists provide immediate glycemic benefit — a potentially synergistic approach for T2D.
Potential in T1D
In preclinical models, menin inhibition promotes β-cell de-repression, proliferation, and insulin production.
This suggests safe, tolerable menin inhibitors could help restore β-cell mass in T1D.
However, autoimmune destruction remains the core driver of T1D and will need to be addressed with complementary immunomodulatory therapies.
TYK2 Inhibitor — First-in-class Oral Therapy for T1D
Clywedog is also advancing the first-in-class, oral, small-molecule TYK2 inhibitor with an outstanding safety and tolerability profile, in clinical development for T1D prevention and early treatment.
Mechanistic Rationale
TYK2 is a genetically validated target in T1D, mediating type I interferon (IFN) and interleukin (IL-12/23) signaling.
Pathogenic TYK2 signaling drives:
Upregulation of HLA class I and chemokines in β-cells
β-cell stress and apoptosis
Priming and activation of autoreactive cytotoxic T cells
Preclinical Data
Clywedog’s nonclinical studies demonstrated that TYK2 inhibition:
Protects human β-cells and islets from IFN-driven damage
Reduces autoimmunity in vitro
Attenuates insulitis in vivo in both prevention and reversal models
Clinical Implication
TYK2 inhibitors offer a dual mechanism: direct β-cell protection plus modulation of autoimmune drivers.
This positions Clywedog’s program as a potential disease-modifying therapy for individuals at risk of, or newly diagnosed with, T1D